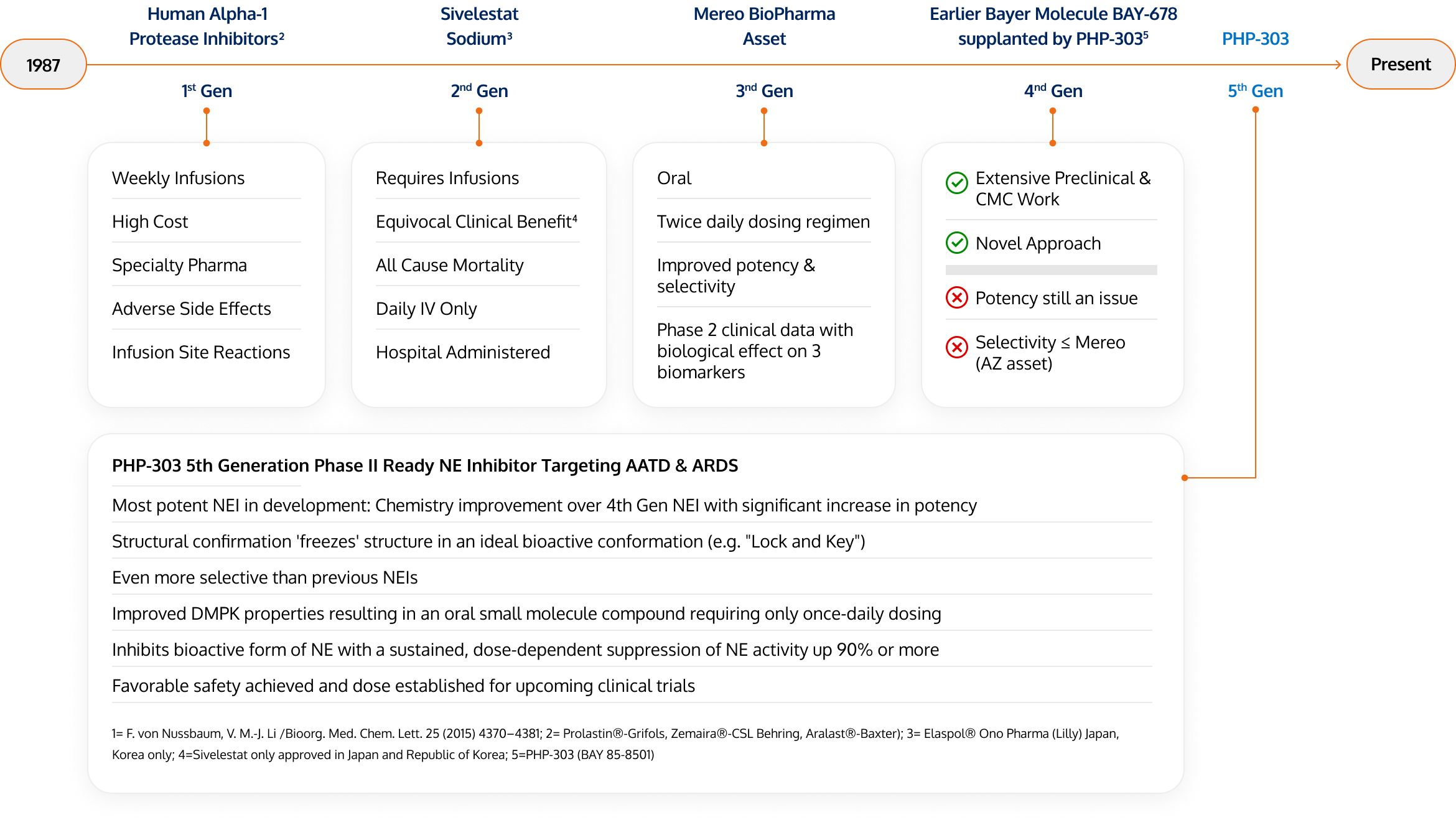
PHP-303
Fifth Generation Phase II Ready Neutrophil Elastase (NE) Inhibitor1 Targeting AATD and ARDS
Alpha-1 Antitrypsin Deficiency (AATD)
What is AATD?
AATD, a potentially life-threatening rare, genetic condition caused by a lack of alpha-1 antitrypsin (AAT) a protein (antiprotease) that protects the lungs from enzymatic degradation by endogenous proteases (such as NE). The disease manifests as early-onset pulmonary emphysema, caused by irreversible destruction of lung tissue supporting gas exchange. PHP-303 is designed to selectively inhibit NE, a neutrophil enzyme, which is the major protease responsible for the destruction of lung tissue in AATD.
What are the current treatment options for AATD?
- Currently no cure for AATD
- Existing therapies attempt to reduce the inflammatory process, reduce exacerbations and the eventual tissue/organ destruction. (E.g. Augmentation/infusions and traditional Bronchodilators, steroids etc.)
- Limitations of current therapies include: 1) low lung penetration, 2) cost 3) inconvenient weekly IV infusions, 4) procurement & 5) pathogen risk
How many people suffer from AATD?
There are an estimated 100,000 patients in the United States and 120,000 patients in Europe with severe AATD.
~120k US/ ~75 EU
Acute Respiratory Distress Syndrome (ARDS)
What is ARDS?
ARDS is a serious lung condition characterized by acute, diffuse, inflammatory lung injury resulting from a range of predisposing etiologies. ARDS generally progresses from a stage of damage to one where “air” (gas) exchange unit becomes compromised, which is followed by a proliferative repair response by the body’s fibroblasts, ultimately leading to lung fibrosis and scarring likely resulting in an increase in morbidity, mortality and healthcare costs.
COVID-19-related ARDS
The emergence of COVID-19 has led to an increase in the incidence of ARDS in a significant number of hospitalized COVID-19 patients, who may also have other significant complications in the renal, cardiovascular, gastrointestinal, and central nervous systems. At this juncture in the pandemic, we are now seeing incidences of not only acute ARDS associated from COVID-19 but also increased reports of “long-COVID”, which we are just beginning to understand. In the future, we will be exploring the potential benefits of PHP-303 treatment across the spectrum of ARDS-related syndromes focusing not only on acute ARDS but also on the more protracted or chronic ARDS-Covid-19 situation.
How many people suffer from ARDS?
The estimated incidence of ARDS in the US ranges from 64.2- 78.9 cases/100,000 people with 75% of these patients presenting with moderate to severe disease. An earlier report (2017) estimated the incidence of ARDS to range from 1.5-79 cases/100,000 people in European countries.
25 days in ICU
& 47 days hospitalized
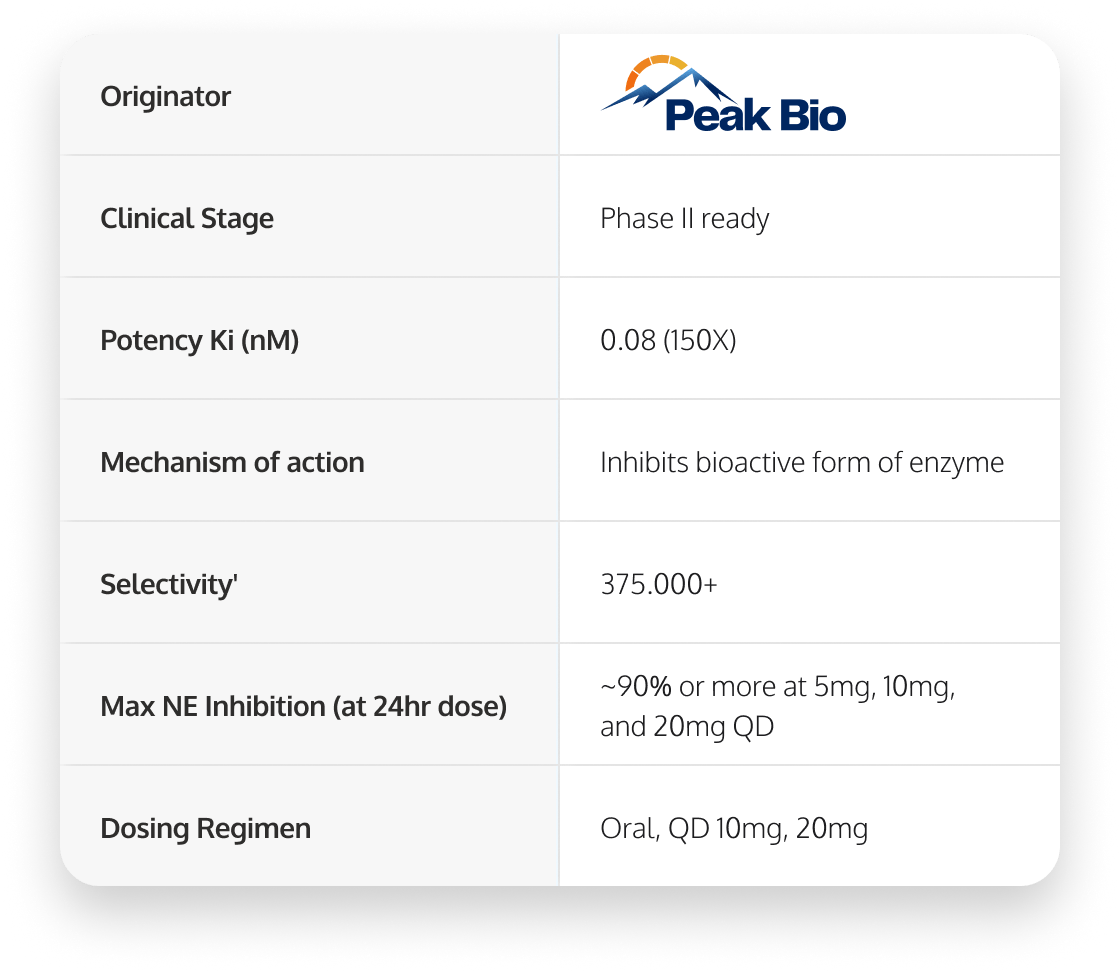
PHP-303
Potentially best-in-class neutrophil elastase inhibitor
PHP-303 is a highly targeted and selective NE inhibitor that shows ~90% NE inhibition at doses of 5mg or higher